This work was carried out in the Laboratory of Organic Chemistry, Helsinki University of Technology, in the years 2001-2006. II Markku Oila designed the research plan with the co-authors, performed the experimental work and interpreted the experimental data.
Synthesis in heterogeneous systems
Solid supported synthesis
It is the formation of a peptide chain, a procedure developed by Merrifield.18 Selected amino acids are coupled with the Merrifield resin 1 to form the polymer-bound 2. The desired product 5 is then cleaved from the resin to the solution by a suitable chemical modification (basic hydrolysis) .
Synthesis on semi-solid supports: nanoparticles
In the first publication of this thesis, an efficient and simple procedure was obtained to form the PyOX- core and, further, to modify the 5-position of the pyridine ring. As previously mentioned (e.g. ch. 3.6.), disymmetric oxazoline ligands can form two different metal complexes, a selectivity feature lacking in e.g.
PyOX preparation procedures from nitriles
Copper activation
A pioneering discovery was made in 1971, after the hydrolysis of picolinonitrile was examined by Breslow et al.24 The copper complexing agent 8 was used as a ligand and the hydrolysis rate of 7 to 10 was examined (Scheme 2).
Zinc activation
Cadmium activation
Substituted PyOX-ligands via nitriles
PyOX preparation by condensation from imidates
Preparation of oxazolines from carboxylic acids
Chlorination of amido alcohol
Other amido alcohol activators in oxazoline synthesis
Dehydrating agents such as phosphorus pentoxide46 or diisopropylcarbodiimide (DIC) with copper(II) triflate47 have been reported, as have sulfuric acid,48 hydrobromic acid49 or perchloric acid.50 Conventional heating (280 °C) is used for the preparation of small oxazolines.46 .
Preparation of other bidentate N, N-oxazolines
It was soon found that the PyrOX core was so much like PyOX that it could also be synthesized from the corresponding β-amido alcohol 33 (Scheme 9) using the well-known chlorination procedure reported for PyOX ligands ( chapter The formed β-chloramide 34 spontaneously cyclizes to oxazoline 35 at room temperature. The first to introduce chiral PyrOX ligands using free pyrrole nitrogen (39) was Brunner in 1998.54 These ligands were synthesized from amino alcohols to be tested in a .PyrOX proved to be a potent catalyst ligand, the search for new analogous structures was introduced.
A few years after Brunner's discovery54, the first attempt to construct a chiral IndOX compound was made.55 The strategy was to cyclize the corresponding amido alcohol using the DAST method (Scheme 11). Nevertheless, rac-44 was found to be a potential antibacterial agent (inhibition of deacetylase LpxC: IC50 = 1.6 µM).55. To date, racemization of the oxazoline ring has been a problem in the rare IndOX syntheses that have been reported, and the first synthesis of enantiopure IndOX compounds is presented in this thesis.
Hydrosilylation: Asymmetric Reduction of Ketones
Diels-Alder-reaction
Meerwein arylation
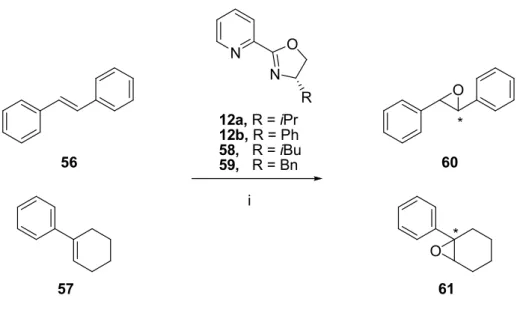
Asymmetric epoxidation
Nucleophilic 1,2-attack on carbonyls
Addition of diethylzinc to aromatic aldehydes
Addition of allyltrialkylsilanes to aromatic aldehydes
Nucleophilic conjugate addition
Michael addition
Another case of Michael additions with PyOX ligands is an approximation made by PyOX ligands with a thioether tail (86), making 86 a tridentate ligand.80 Scheme 19 shows this approximation, giving 87 a selectivity of up to 19% ee .
Allylic substitution
A more recent asymmetric substitution reaction performed with PyOX is the Pd-catalyzed allylic substitution reaction by Trost.85 It involves the alkylation of chalconol acetate 90 with dimethyl malonate (Scheme 21). It has been used for PyOX-bidentate ligands and some tridentates27 or for polymer-bound tridentate ligands86 by Moberg and for fused PyOX-28 pyridine ring ligands or 4,6-substituted pyridine ring ligands87 by Chelucci, all of which qual. excellent at up to 99% ee for tridentate PyOX ligands)27. This procedure was carried out by deprotonating dimethyl malonate and adding it to the solution of the formed complex of the alipalladium catalyst and 90 (Scheme 21).
Since the reactive allylic intermediate of substrate 90 (1,3-diphenylallyl cation) is symmetrical, these four reaction centers (Scheme 22) can form only two products.
Cyclopropanation of olefins
The Evans procedure has been applied to more hindered olefins and more electron-poor olefins to find the limitations of this reaction.89 Bi- and tridentate P,N and P,N,N ligands with ruthenium catalysts have also been used for cyclopropanation.90. Chelucci investigated the use of PyOX catalysts (e.g. 97) in the asymmetric cyclopropanation of styrene 36 using ethyl diazoacetate (38, Scheme 23).91 Like Evans88, he used copper(I) triflate as the catalyst metal salt to obtain moderate selectivities range (up to 60% ee) with PyOX ligands. This was a notable improvement over the Evans C2 symmetric BOX catalyst in the case of styrene (Scheme 24).
Due to an increasing demand for enantiopure compounds, it is important to develop new methods to induce this purity in an efficient manner. In this thesis, the strategy was primarily to prepare bidentate chiral PyOX ligands in an efficient way to induce chiral information in an asymmetric test reaction. For this reason, the goal was also set in preparing new gold nanoligands with the PyOX functionality and testing their catalytic activity.
Finally, to address the problems with a general oxazoline formation procedure, my procedure was used to develop an efficient synthetic route for a previously never enantiopurely synthesized bidentate N,N-oxazoline compound family, the 2-indolyloxazolines (IndOX).
Synthesis of a mercapto derived soluble PyOX-ligand (I)
Amidoalcohol 100 was mesylated to form the isolable mesylate precursor 106.12 To our knowledge, a one-step cyclization using tosylation has only been reported by Meyers, using extended reaction times and mixing. elevated temperature12 to achieve a clean reaction. S -Trityl-protected mercaptoethanol 10994 was added to acid 99 according to standard esterification procedures to form the target protected molecule 110 ( Scheme 27 ). It was carried out using TFA with triethylsilane as a scavenger of the trityl cation formed.95 Addition of Et3SiH required.
The result of this synthesis is of great value due to the combination of the efficient preparation of a chiral ligand and the new approach to form the terminal mercapto in a ligand such as 98. Mercapto adducts are of great interest because of their well-known assocn. on gold surfaces96 or nanoparticles.97 Applications involving such complexes are explored at an increasing rate,98 making efficient mercapto-derived ligands valuable in e.g.
Ligand preparation via linking: A new approach to solid-phase synthesis (II)
The synthesis of 111 began by examining the corresponding solution-phase reactions ( Scheme 29 ) by IR analysis as a reference for solid-phase follow-up. The protocol for Boc removal was originally adapted for solid-phase synthesis and is thus also used in this model test.99. The key observation in the presented synthetic route was the benzylation step, as it turned out that the use of cesium carbonate in step (iii) (Scheme 29) instead of potassium carbonate decomposed 113 and formed 115, giving a complex mixture of products . 100 The use of potassium carbonate accordingly gave clean conversion to acetone, as previously reported. 80 Since acetone is not a compatible solvent with Merrifield resin due to poor swelling, racemization and decomposition with potassium carbonate in DMF was also tested in my study and found to cause none of these.
Each of the reactions was followed and monitored by FTIR analysis after isolation of an analytical sample to form the new amino alcohol linker 111 (Scheme 31). Further functionalization at the pyridine ring was performed by designing acid 124 by Sonogashira-type coupling of compounds 135 and 136.102 A notable problem was that the use of copper (original Sonogashira conditions)103 in different ratios totally inhibited this coupling, in contrast to previous studies .104. The approach presented in Scheme 32 is, to the best of my knowledge, the first approach to form PyOX ligands via simultaneous binding and ligand formation.
The general way to prepare solid-supported ligands is to first prepare the soluble ligand and then attach it to the support.
Chirally modified gold nanoparticles (III)
The long reaction time was a surprise in this synthesis, and after 20 days particle 138 could not be isolated at all, indicating the presence of larger and more soluble particles or conglomerates in solution. After the precipitation of 138, its composition was analyzed by CHN-analysis and the Au : S : N ratio was obtained, indicating that half of the original hexanthiolate ligands of 137 were substituted by 98. Furthermore, this would mean that all thiolates were on the surface of the hollow gold core , an observation that was supported by analysis of the ESCA compound.
The big observation, however, was the size of 138: TEM images of these particles (b, Figure 7) showed that a diameter of 1.2 ± 0.2 nm was reached, i.e. smaller than ever in this field! Previous modeling studies106 predicted that this would correspond to a fullerene-like core, consisting of 32 gold atoms. Because 138 was a chiral ligand, it was used in an asymmetric test reaction by Trost (Scheme 21)85 and gave selectivities slightly worse than the soluble analogues, but better than the corresponding polymer-bound adduct (presented in section 5.2).
The asymmetric Henry reaction – a new Application for PyOX (IV)
Finally, this paper yielded a chiral PyOX-derived gold nanoparticle 138 , to the best of my knowledge, of a size never before achieved in experimental chemistry. Moreover, the reactions were quite clean and inexpensive starting materials and solvents were used, which is of industrial interest. The asymmetric Henry reaction involves a little more consideration, as the mechanism is not yet fully understood.
In Scheme 35, it can be seen that the formation of the intermolecular ring in 142 is essential for product formation. To test the Henry reaction with PyOX-ligands, a low-electron aldehyde ( p -nitrobenzaldehyde, 71 ) was chosen due to its good reactivity to effectively monitor the selectivity of different PyOX-ligands ( Scheme 36 ). The yields and selectivities were monitored in these test reactions and the best ligand (147, complete conversion, 70% ee) was selected and fixed for the next series of reactions where the aldehydes were varied (Scheme 37).
Preparation of a Novel Ligand Family: The IndOX (V)
In the study by Pfaltz,109 attempts were made to form the corresponding indolyl ligand species (IndOX). In the last part of my thesis, a protocol to form a chiral IndOX compound family was reported. The indolyl-2-carboxylic acids 154–156 (Scheme 39) were chosen to vary the substituent at the 5-position of the indolyl ring from electron donating to withdrawing.
To ensure the optical purity of the oxazoline ligands, the ( S )-enantiomer 168 was also prepared and purified by chiral HPLC. The key issue was therefore to develop an efficient method that is mild enough not to racemize the resulting oxazolines and that also offers the possibility of modifying both rings of the compound core. To further extend the synthetic value of the optimized protocol for the formation of PyOX, the pyridine ring was replaced by an indole ring and a new family of chiral ligands, IndOX, was synthesized by changing the substituents of both rings of its core.
Furthermore, a new catalytically asymmetric application for most of the new oxazoline compds., viz.